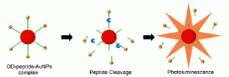
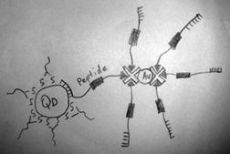
A) The idea of such biosensor has been recently published by researchers from Rice university (see E. Chang, J.S. Miller, J.T. Sun, W.W. Yu, V.L. Colvin, R. Drezek, J.L. West, Protease-activated quantum dot probes, Biochem. Biophys. Res. Comm., v. 334 (4), pp. 1317-1321 (2005)).
B) Presence of Au nanoparticles in the proximity of fluorescent quantum dots results in the quenching of fluorescence.
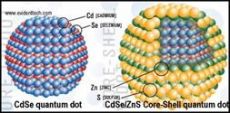
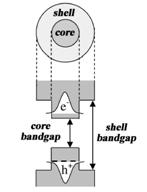
C) Quantum yield of fluorescence is too low for uncoated quantum dots because electrons excited into conductance band have high probability of hitting a solvent or stabilizer molecule just outside the nanoparticle. Such events result in a non-radiative loss of energy and decreased quantum yield. If CdSe is coated by a larger-bandgap semiconductor, excited electrons are localized in the core because conductance band of the core has lower energy (see schematic below). This dramatically reduces non-radiative loss of energy due to collisions with the outside molecules and increases the quantum yield.
E) There are many possibilities, any correct route is acceptable. Points to watch for: if you decided to synthesize core-shell quantum dots instead of buying them, be sure to include hydrophilization step: synthesis of fluorescent quantum dots is performed in hydrophobic medium (using TOPO as a solvent), and they would not form a stable suspension in water unless hydrophilized (e.g., by mercaptoacetic acid, but many other ways are available).
F) The biosensors prepared as described would not be able to reach the tumor site(s). They will be quickly uptaken by reticuloendothelial system (RES) in vivo and will end up in liver in few minutes. To prolong their half-life in the circulation and mask them from the RES, additional coating by polyethyleneglycol is required. In addition, since red light is easily adsorbed by the tissues, fluorescence would not be easily detectable in a live mouse. Use of quantum dots that fluoresce in the near-infrared part of the spectrum should be considered because absorption of near infrared radiation by tissues is much weaker.
G) Although solubility of CdSe is quite low and CdSe-containing nanoparticles were shown to have low short-term toxicity, it is unlikely they will ever be used in vivo in human patients. The reason is that long term degradation pathway for these nanoparticles in not known and all soluble cadmium and selenium compounds are known to be highly toxic. Very long term studies (tens of years) on large number of volunteers are needed to determine the safety of these nanoparticles; however the risk of such studies is too high and it is unlikely they will ever be conducted.
В качестве более подробного решения в данной задаче размещается ответ на задачу одного из участников (Е.Г.Евтушенко), поскольку оно лучше и подробнее стандартного решения, предоставленного автором, кроме того, это единственное решение, получившее максимальное количество баллов.
A) Explain the principle of work of this biosensor
This biosensor utilizes the quenching of quantum dot fluorescence by a proximal gold nanoparticle. Due to biologists are not experts in physics, I can’t give a strict physical explanation of this phenomenon. Briefly, four models were suggested:
1) Förster dipole−dipole resonance energy transfer (FRET), with r-6 distance dependence;
2) Nanosurface energy transfer (NSET), which is similar to FRET, following a r-4 distance dependence;
3) Dipole−metal particle energy transfer;
Or even
4) Charge transfer, when Au NP donates an electron to valance band of excited QD with near exponential distance dependence.
Experiments show that such interactions extend significantly beyond the classical Förster range.
This phenomenon could find a practical application in many fields of nanotechnology, like nanoscale rulers, sensing of molecular interactions or enzymatic activity sensors. The latter should consist of QD and Au NP, joined with short cleavable linker. The enzyme (e.g. protease) with substrate specificity to linker sequence cleaves the linker, causing the restoration of fluorescence. If such protease is expressed by some cell types, this nanobiosensor could be used to visualize such cells.
B) Why fluorescence is not observed in the case of normal cells?
As it was noted in the statement of a problem, normal cells don’t express protease, specific to linker sequence and QD fluorescence remains quenched.
C) Explain why uncoated CdSe quantum dots cannot be used instead of core-shell CdSe/ZnS quantum dots.
Uncoated CdSe QDs contains structural defects, such as vacancies, local lattice mismatches, or dangling bonds at the surface. This defects act as effective traps for both holes and electrons in excited QD. The recombination of trapped charge carriers occurs in a nonradiative way. Thus, quantum yield of uncoated QDs is very low. If the CdSe core is coated with wide bandgap semiconductor, like ZnS, both electrons and holes remain localized in a core. At the same time structural defects are compensated. As a result quantum yield rises considerably. Even better quantum yield could be achieved with CdSe/ZnxCd1-xS (x = 0.7, approx. 5 monolayers of ZnxCd1-xS alloy) core-shell QDs.
D) What can you tell about amino acid sequence of the peptide used for the attachment of Au nanoparticles to core-shell CdSe/ZnS quantum dots?
First, it should contain a specific sequence, a substrate for cancer cell expressed protease. Second, to be specific, this sequence should not contain cleavage sites for other possible proteases. Furthermore, peptide sequence should not be a partner for any strict biomolecular interaction, because it could sterically block the cleavage by target protease. Third, peptide sequence must not exceed the energy transfer distance necessary for AuNPs to suppress QD fluorescence. Designing peptide sequence, we should take into account possible α-helical or any other secondary structure of the peptide, which shortens effective peptide length. We could use it, or we could disrupt it by introduction of several glycines. Glycine residue is very flexible and incompatible with α-helix.
Forth, if we use a dihydrolipoic acid-PEG capped QDs, we could utilize a self-assembly strategy for peptide attachment to QD. In this case peptide should have so called basic leucine zipper or polyhistidine tag (His6) on its end.
E) Describe a detailed synthetic route to prepare the nanodevices described above. You can use any commercially available reagents (for uncommon reagent, please provide the name of the supplier and verifiable catalog number).
To synthesize the nanobiosensor we will use the following strategy:
1) Synthesize TOP/TOPO-capped CdSe/ZnS Core/Shell QDs;
2) Exchange native TOP/TOPO ligands with dihydrolipoic acid (DHLA)-PEG. Such coating results in water-soluble, highly fluorescent and highly stable QDs with reduced nonspecific binding to cells;
3) Buy an engineered peptide, containing His6 tag on C-terminus and biotin on N-terminus;
4) Buy streptavidin-coated 2 nm Au NPs (typically 3-4 streptavidin molecules per NP);
5) Conjugate peptide with Au NPs using biotin-streptavidin interaction;
6) Fluorometrically titrate DHLA-PEG QDs with Au-NP-Peptide conjugate until total quenching of fluorescence. Peptide will attach to QD via His6 tag.
Detailed protocol:
1) Synthesis of CdSe/ZnS Core/Shell QDs.
Se precursor, tributylphosphine selenide (TBP-Se) is prepared by dissolving 0.15 mmol of selenium shot in 100 mL of TBP under inert atmosphere and stirring vigorously overnight, forming a
A Cd+Se precursor solution containing 0.5 mmol cadmium 2,4-pentanedionate (Cd(acac)2), 0.25 mL dodecanal (DDA), and 2.8 mL tri-n-octylphosphine (TOP) should be degassed at
The pellet is re-dispersed in hexane, filtered through a 0.2 μm filter, and injected into a degassed solution of
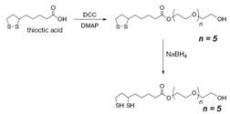
2) Synthesis of capping ligand dihydrolipoic acid (DHLA)-PEG.
First stage: a round bottomed flask is filled with thioctic (lipoic) acid (
Second stage: a round bottomed flask is filled with the pegylated lipoic acid derivative (1 mmol), ethanol (10 mL), and water (2 mL). NaBH4 (150 mg, 4 mmol) is added and the reaction is heated gently until the yellow color vanished to give a cloudy colorless solution. The mixture is diluted with CHCl3 (200 mL), dried over MgSO4, filtered and evaporated to give a colorless oil.
3) The replacement of native TOP/TOPO ligands with DHLA-PEG.
0.2 mL QDs in growth solution are precipitated by adding 2 mL of methanol, centrifuged at 3000g. Clear supernatant is discarded; the precipitate is washed by 0.5 mL methanol and centrifuged again. QDs are dried using N2.
50 μL of DHLA-PEG and 10 μL of methanol are added to 10 mg of dry QDs. The mixture is stirred gently under N2 at
4) Engineered peptide
Peptide, containing His6 tag on C-terminus and biotin on N-terminus could be purchased from Elim Biopharmaceuticals, Inc.
5) Streptavidin-coated Au NPs
Highly spherical, non-aggregated Au NPs with narrow size distribution, with streptavidin corona could be purchased from BBI.
Catalog numbers are EM.STP2, EM.STP5, EM.STP10 and EM.STP15 for 2, 5, 10 or 15 nm Au NPs. The optical density at 520 nm varies from 3.1 to 15 depending on NP’s size.
6) Preparation of peptide-AuNP conjugate.
0.2 mL of 1.5 μM streptavidin-coated 2 nm Au NPs in PBS (pH = 7.4) is incubated with 0.2 mL of 14 μM biotinilated peptide for 1 h. Excess peptide is removed by ultrafiltration on Vivaspin-6 using 10 000 MW cutoff filter. Additionally, we have a possibility to recover the excess peptide using His6 tag.
7) Titration of DHLA-PEG-capped QDs with peptide-AuNP conjugate.
10 μL of 8 μM DHLA-PEG-capped QDs in PBS (pH = 7.4) is placed in Corning Black 384-Well Polypropylene Assay Plate. 10 μL portions of 1.5 μM peptide-AuNP in PBS (pH = 7.4) are added with subsequent 30 minutes incubation and fluorescence measurements using FLUOstar Omega microplate reading fluorometer. Synthesis is finished when the fluorescence is totally quenched. Biosensor conjugate should be purified using gel filtration chromatography in order to remove aggregates (binding of His6 to QDs is stochastic, so we have two multivalent partners and minor amounts of polymer could occur as a result) and free Au NPs.
Possible structure of the sensor is shown on the left picture. If we want to obtain the sensor with better sensitivity, we could use concurrent principle in stage 6, mixing biotinilated peptide with free biotin in defined ratio. Using this approach, we can decrease the average number of peptides down to 2-3 per Au NP. The number of biotin binding sites per Au NP could be determined using fluorometric titration with biotin-FITC.
F) Researchers decided to use the system described above to monitor the progression of metastasis for a squamous cell carcinoma in a mouse model. What modifications should be done to the sensor so that it could be used for this in vivo study?
Biological tissues are not transparent in visible region, but they have a “transparency window” in near infrared (NIR) region, 700-1000 nm. NIR radiation penetrates few centimeters deep into the tissue. Thus, we have to change visible fluorescent CdSe QDs to NIR fluorescent PbS, PbSe, PbTe or HgCdTe QDs, combining such core with appropriate wide bandgap semiconductor.
G) Would you recommend in vivo use of this biosensor in human patients? Provide your arguments.
This biosensor is a perfect tool for visualization of cancer cells in biopsy, but I’m afraid it will be not so efficient in vivo. Although NIR region is called a “transparency window”, the biological tissue is not so transparent to allow NIR radiation penetration trough the whole human body. So, deep tumors can’t be visualized. The second reason is the cytotoxicity of small Au NPs. It was shown, that certain cells could uptake 2 nm Au NPs. These NPs alter the expression of genes involved in processes of transcription, cell growth, and response to viruses. Also the products of QDs degradation, such as cadmium, lead, mercury, selenium and tellurium ions are highly toxic.